S. boulardii CNCM I-745 is supported by numerous key clinical studies that demonstrate its efficacy in the management of diarrhea
- 10 min
The efficacy of Saccharomyces boulardii CNCM I-745 is supported by extensive clinical evidence
The efficacy of S. boulardii CNCM I-745 has been investigated in numerous clinical studies since it was first isolated. At present, there have been 100 clinical trials investigating S. boulardii CNCM I-745. From these 100, several key meta-analysis that have reviewed the literature demonstrating the efficacy of S. boulardii CNCM I-745 in multiple gastrointestinal disorders are described here.
Key studies investigating Saccharomyces boulardii CNCM I-745 to prevent antibiotic-associated diarrhea
Szajewska, H et al. 2015.
Systemic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea.1
https://pubmed.ncbi.nlm.nih.gov/26216624/
I. Introduction
This meta-analysis investigated the effectiveness of probiotics in preventing antibiotic-associated diarrhea in children and adults.
II. Design
A database search for randomised controlled trials (RCTs) was performed.
Inclusion criteria for studies: RCTs investigating adults and children who received antibiotic therapy, and where experimental arms received S. boulardii at any dose/duration; or placebo/no treatment for control group.
III. Outcomes
In this meta-analysis, the outcomes are:
A. Primary outcomes: measurement of incidence of diarrhea or antibiotic-associated diarrhea.
B. Secondary outcome: measurement of incidence of C. difficile-associated diarrhea.
IV. Results
There were 21 studies analysed that included 15 trials in adults and 6 trials in children, totalling 4780 participants (2441 treated with S. boulardii and 2339 in the control group).
A. Primary outcomes:
- Diarrhea: for every 10 patients receiving daily S. boulardii with antibiotics, 1 fewer would develop diarrhea (NNT: 10, 95% CI: 9-13).
- Antibiotic-associated Diarrhea:
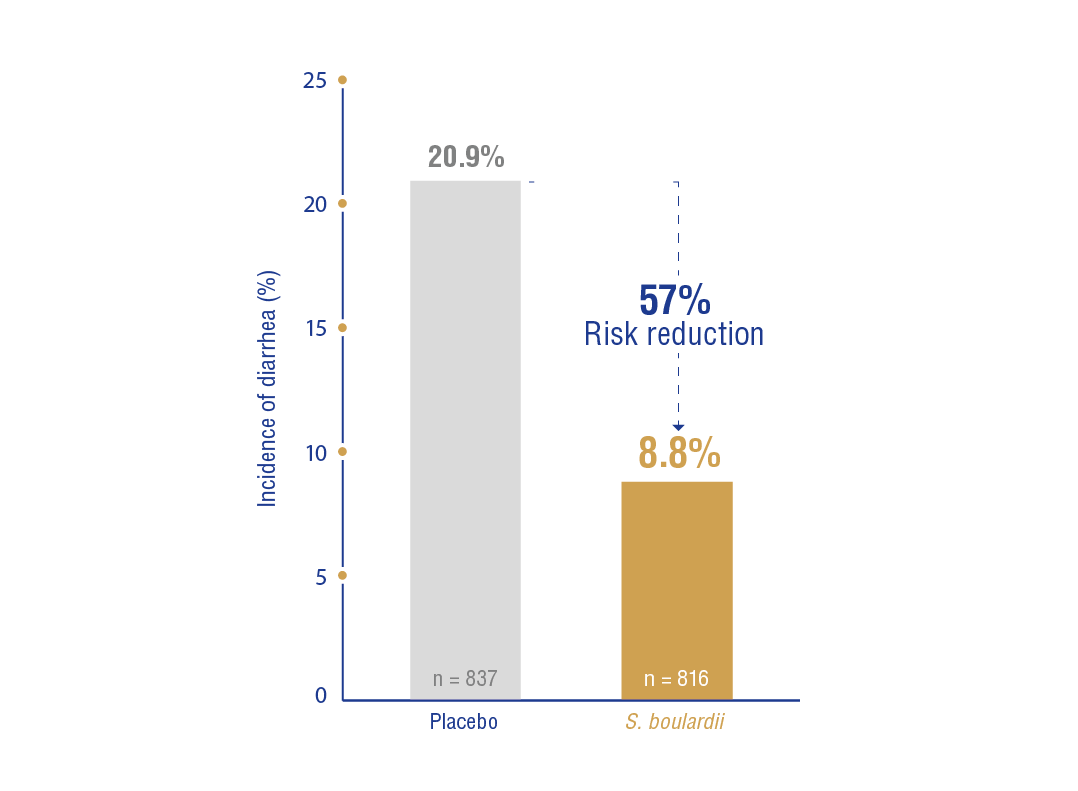
a) Children: In 6 RCTs (n = 1653), treatment with S. boulardii vs. placebo or no treatment reduced the risk of diarrhea from 20.9% to 8.8% (RR: 0.43, 95% CI: 0.30-0.60, NNT: 9, 95% CI: 7-12) (Figure 1.1).
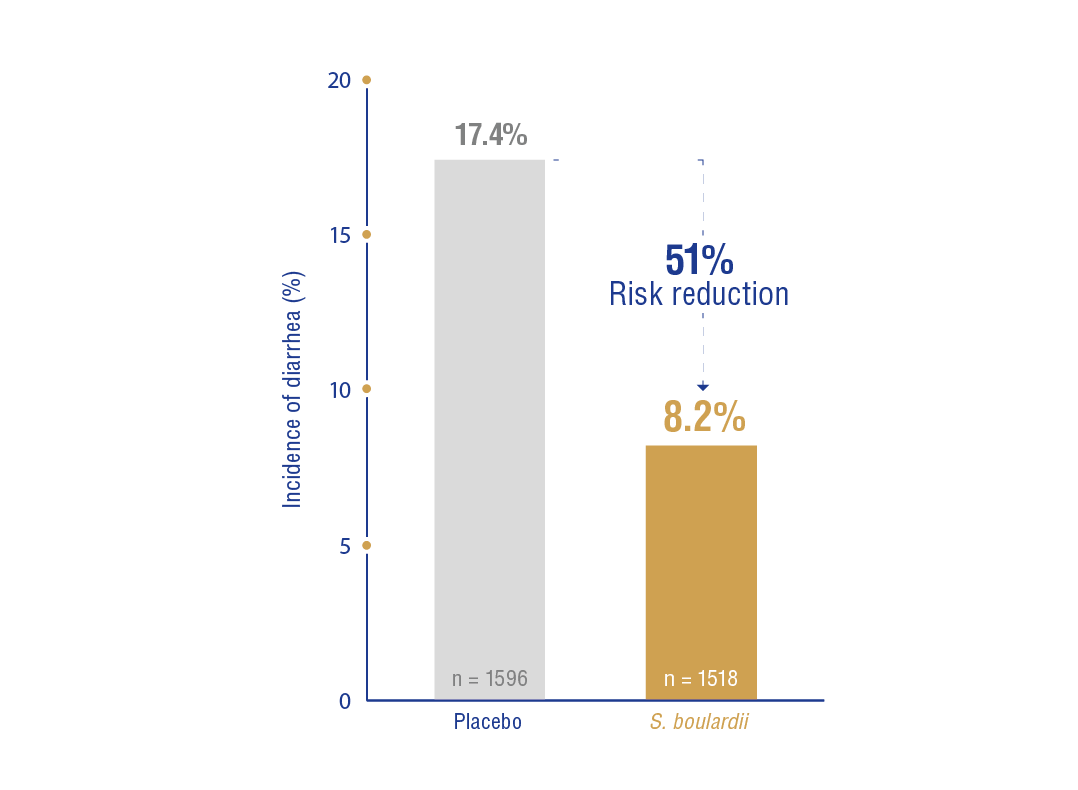
b) Adults: In 15 RCTs (n = 3114), treatment with S. boulardii vs. placebo or no treatment reduced the risk from 17.4% to 8.2% (RR: 0.49, 95% CI: 0.38-0.63, NNT: 11, 95% 9-15) (Figure 1.2)
- Overall:
B. Secondary outcomes:
C. difficile-associated diarrhea: Sub-group analysis demonstrated that treatment with S. boulardii reduced the risk of C. difficile–associated diarrhea in children (2 RCTs, n = 579, RR: 0.25, 95% CI: 0.08-0.73).
V. Conclusion
Key meta-analysis investigating Saccharomyces boulardii CNCM I-745 to prevent Helicobacter pylori eradication therapy-associated gastrointestinal side effects
Zhou B, et al. 2019.
Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta‐analysis with trial sequential analysis.2
https://pubmed.ncbi.nlm.nih.gov/31414551/
I. Introduction
This meta-analysis investigated the effectiveness of S. boulardii CNCM I-745 on H. pylori eradication rates and adverse effects (including diarrhea) in adults and children.
II. Design
A database search for randomised controlled trials (RCTs) was performed.
Inclusion criteria for studies: RCTs investigating S. boulardii as an adjuvant therapy for H. pylori eradication in adults and children.
III. Outcomes
In this meta-analysis, the outcomes are:
A. Primary outcome: H. pylori eradication rates.
B. Secondary outcomes:
- Total adverse effects
- Specific adverse effects
- Need for discontinuation
IV. Results
Studies reviewed:
18 randomized controlled trials (from 2002-2019) with:
- 21 intervention arms.
- 3592 participants.
A. Primary outcomes:
- Helicobacter pylori eradication rates:
B. Secondary outcomes:
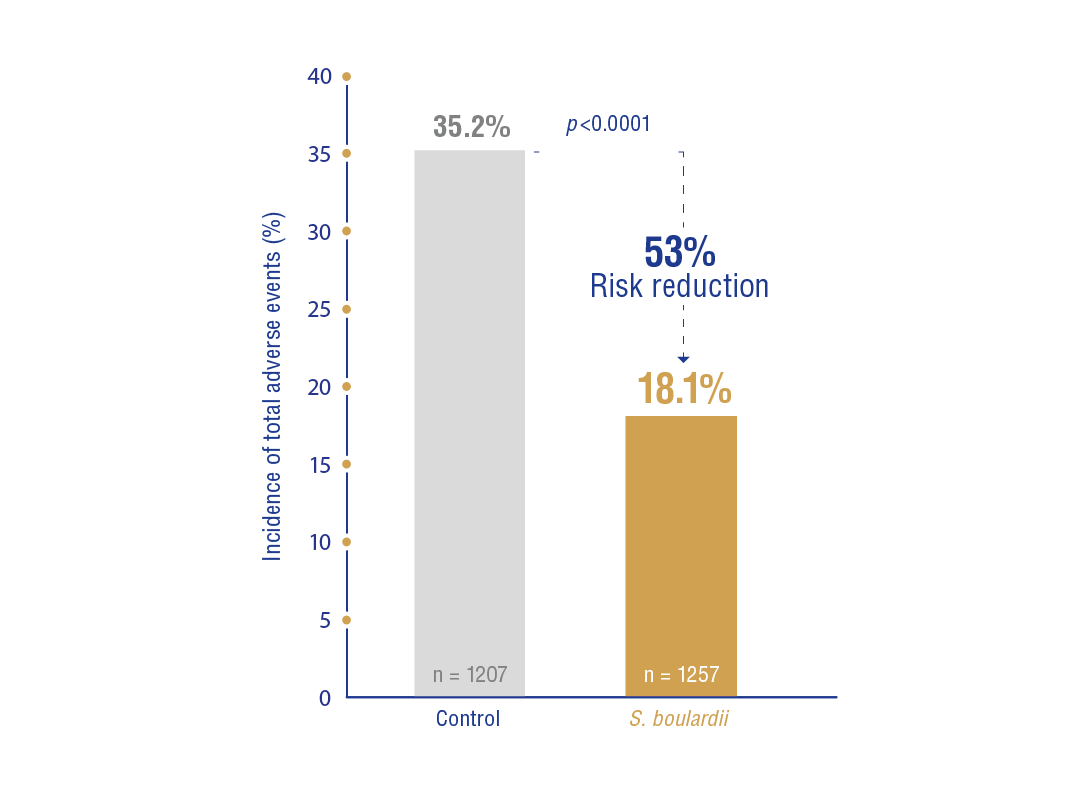
- Total adverse events:
(18.1%) vs. control patients (35.2%); RR = 0.47, 95% CI: 0.36-0.61; p < 0.0001) (Figure 2).
- Specific adverse events: S. boulardii significantly reduced the incidence of diarrhea, nausea, constipation, and abdominal distention.
- Discontinuation: S. boulardii significantly reduced the need for discontinuation (1.6%) vs. the control group (4.9%; RR = 0.33; 95% CI: 0.16-0.69; p = 0.003).
V. Conclusion
Compared with standard eradication therapy alone,
Key meta-analysis investigating Saccharomyces boulardii CNCM I-745 to prevent Clostridium difficile disease
Szajewska, H et al. 2015.
Systemic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhea.1
https://pubmed.ncbi.nlm.nih.gov/26216624/
I. Introduction
This meta-analysis investigated as a secondary outcome the effectiveness of Saccharomyces boulardii in preventing recurrent C. difficile-associated diarrhea in children (sub-analysis).
To see the summary of the entire study including study design and primary outcomes please refer to the section antibiotic-associated diarrhea developed at the beginning of the article.
II. Design
A database search for randomised controlled trials (RCTs) was performed.
Inclusion criteria for studies: please refer to the section “Antibiotic-associated diarrhea” at the beginning of the page.
III. Outcomes
A. Primary outcomes: please refer to the section “Antibiotic-associated diarrhea” at the beginning of the page.
B. Secondary outcomes: measurement of incidence of C. difficile-associated diarrhea.
IV. Results
A. Studies reviewed:
- 21 studies, 12 of them were placebo controlled.
- 15 trials in adults, 6 trials in children.
- 4780 participants (2441 treated with S. boulardii and 2339 in the control group)
B. Primary outcomes:
- Diarrhea: For every 10 patients receiving daily S. boulardii with antibiotics, 1 fewer would develop diarrhea (NNT: 10, 95% CI: 9-13).
- Antibiotic-associated Diarrhea:
a) Children: In 6 RCTs (n = 1653), treatment with S. boulardii vs. placebo or no treatment reduced the risk of diarrhea from 20.9% to 8.8% (RR: 0.43, 95% CI: 0.30-0.60, NNT: 9, 95% CI: 7-12).
b) Adults: In 15 RCTs (n = 3114), treatment with S. boulardii vs. placebo or no treatment reduced the risk from 17.4% to 8.2% (RR: 0.49, 95% CI: 0.38-0.63, NNT: 11, 95% 9-15).
- Overall: Treatment with S. boulardii vs. placebo or no treatment reduced the risk of AAD in patients (children and adults) treated with antibiotics from 18.7 to 8.5% (RR: 0.47, 95% CI: 0.38-0.57, random effect model), with a reduction of risk by 53%
C. Secondary outcome:
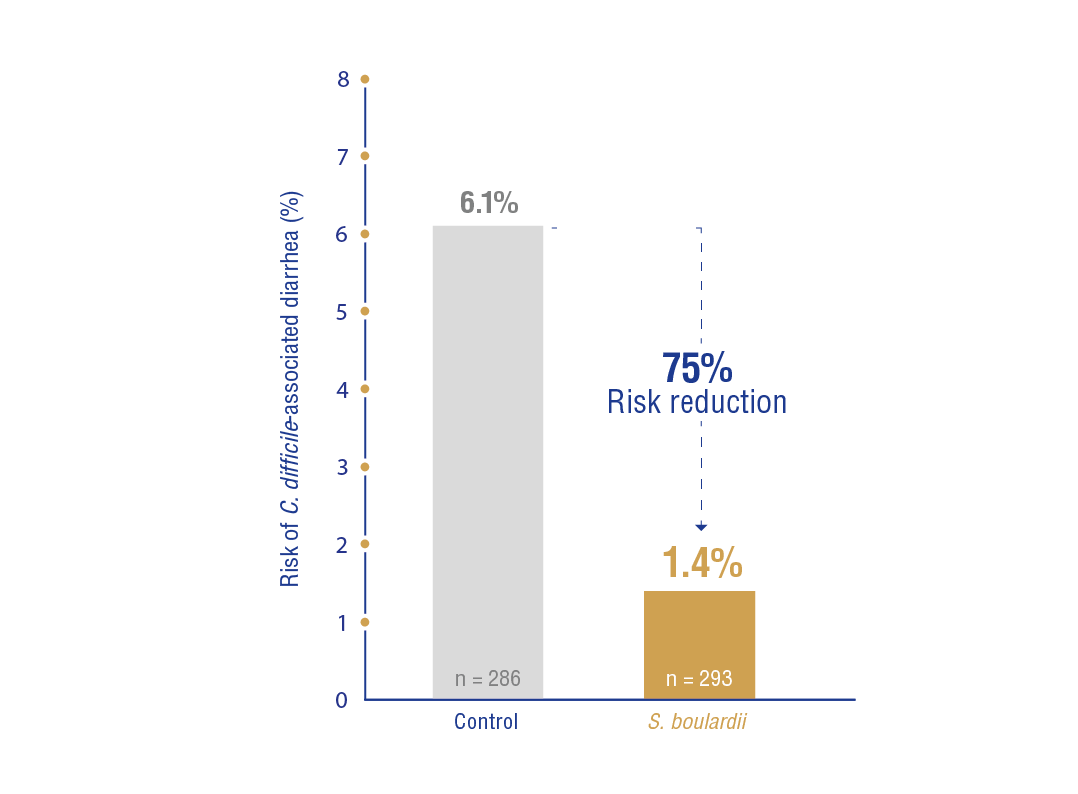
C. difficile-associated diarrhea: Sub-group analysis demonstrated that
(2 RCTs, n = 579, RR: 0.25, 95% CI: 0.08-0.73) (Figure 3).
V. Conclusion
McFarland, LV. 2006.
Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease.3
https://pubmed.ncbi.nlm.nih.gov/16635227/
I. Introduction
This meta-analysis investigated the efficacy of probiotics to prevent antibiotic-associated diarrhea and Clostridium difficile infections following antibiotic use.
II. Design
A database search for randomised controlled trials (RCTs) investigating the use of probiotics to prevent AAD and treatment of C. difficile disease (CDD) was performed.
Inclusion criteria for studies : Randomised, controlled, blinded efficacy trials.
III. Outcomes
A. Primary outcomes:
- Antibiotic-associated diarrhea (within 2 months of antibiotic exposure)
- Clostridium difficile disease (new episode of diarrhea associated with a positive culture or toxin (A or B) assay within 1-month exposure to antibiotics).
B. Secondary outcome:
- Prevention of Clostridium difficile disease
IV. Results
A. Studies reviewed:
n = 25 studies (2810 participants)., including 6 S. boulardii studies.
B. Effect of interventions:
- Antibiotic-associated diarrhea:
(Combined RR: 0.37; 95% CI: 0.26-0.52; p <0.0001).
- Clostridium difficile disease:
Combined effect for all probiotics assessed showed a significant protective effect for CDD (RR: 0.59; 95% CI: 0.41-0.85; Z = 2.8; p = 0.005) (Figure 4).
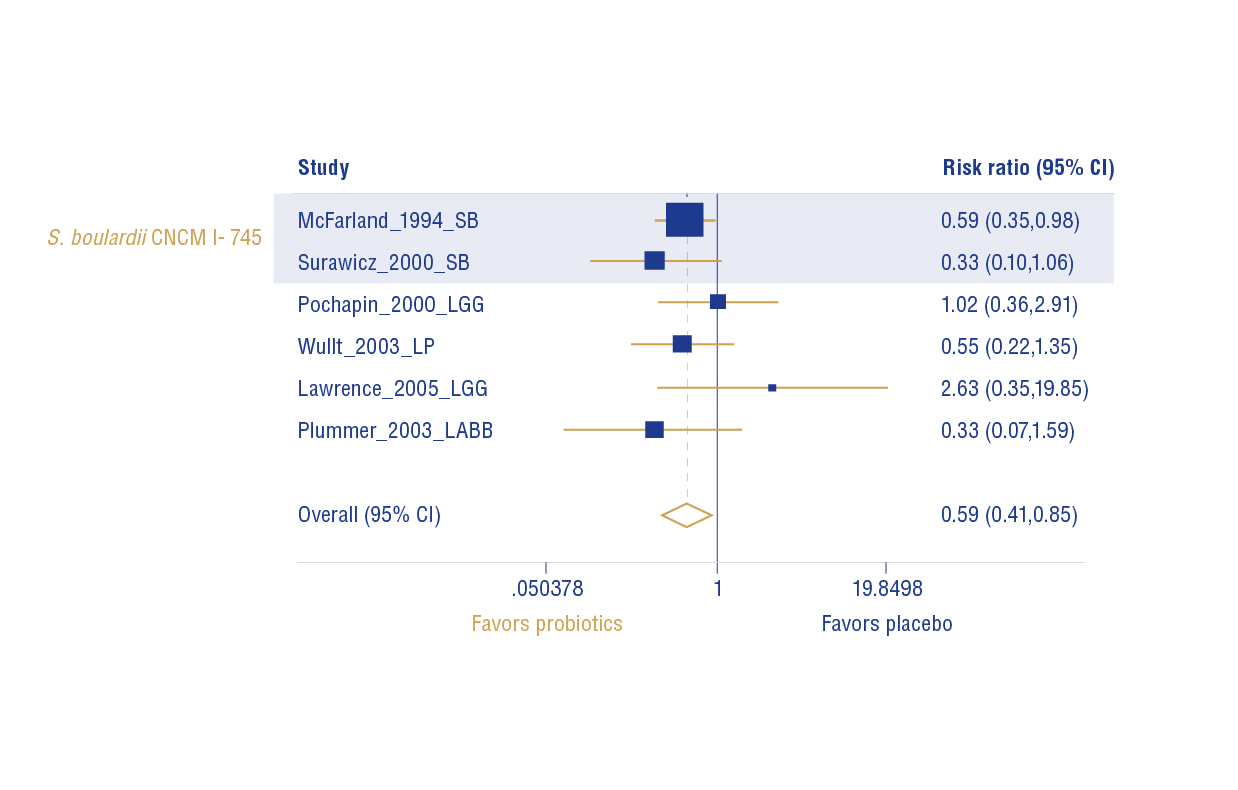
SB = Saccharomyces boulardii, LGG = Lactobacillus rhamnosus GG, LP = Lactobacillus plantarum 299v, LA = Lactobacillus acidophilus; BB = Bifidobacterium bifidum.
V. Conclusion
Key studies investigating Saccharomyces boulardii CNCM I-745 to treat acute diarrhea
Szajewska H, et al. 2020.
Systematic review with meta-analysis: Saccharomyces boulardii for treating acute gastroenteritis in children-a 2020 update.4
https://pubmed.ncbi.nlm.nih.gov/32056266/
I. Introduction
This meta-analysis investigated the efficacy of S. boulardii treatment for acute diarrhea in children.
II. Design
A database search for randomised controlled and non-randomised trials (RCTs) investigating S. boulardii in childhood acute diarrhea was performed.
Inclusion criteria for studies: RCTs with children aged 1 month to 15 years with acute gastroenteritis.
III. Outcomes
In this meta-analysis, the outcomes are:
- Duration of diarrhea
- Stool volume
IV. Results
A. Studies reviewed:
- n = 23 studies, with 3450 participants
- Patients aged 1 month – 15 years (21 studies with children aged ≤60 months).
B. Outcomes:
(mean difference = –1.06 days; 95% CI: −1.32, −0.79; high heterogeneity [I2 = 90%]; very low quality of evidence).
Most of the RCTs included in the study used Saccharomyces boulardii CNCM I-745 strain. (Figure 5)
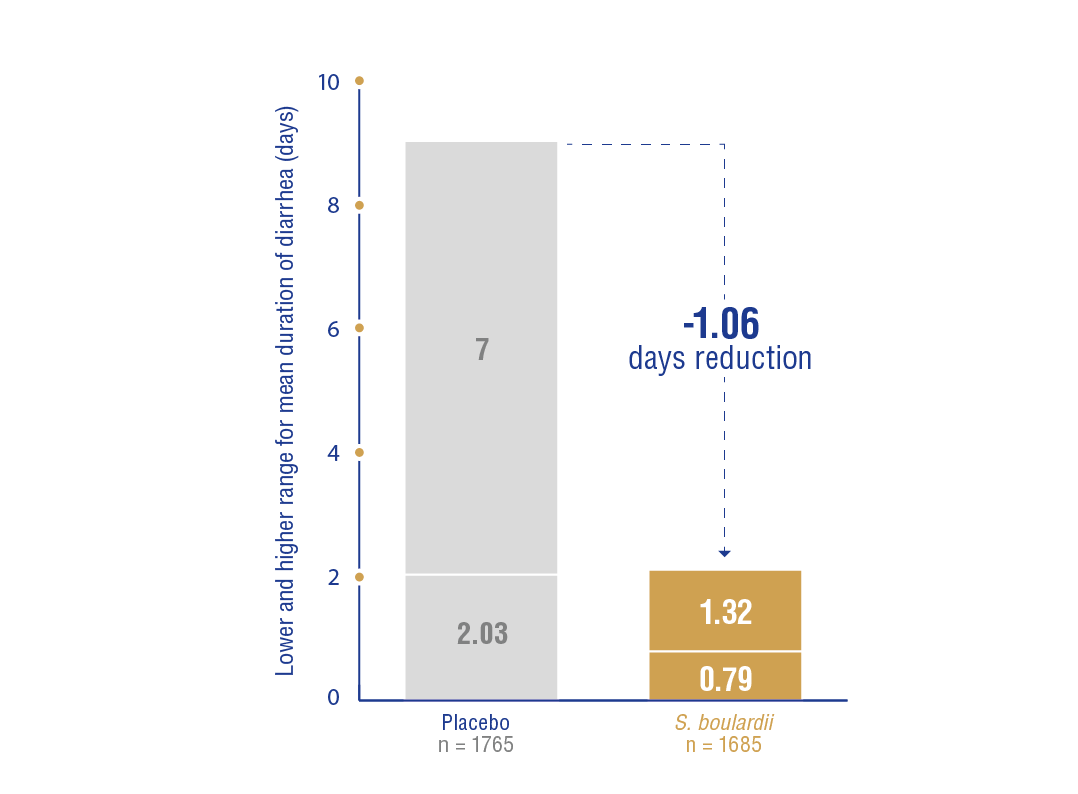
None of the included trials assessed the effect of S. boulardii on stool volume.
V. Conclusion
Feizizadeh S, et al. 2014.
Efficacy and safety of Saccharomyces boulardii for acute diarrhea.5
https://pubmed.ncbi.nlm.nih.gov/24958586/
I. Introduction
This meta-analysis investigated the efficacy of S. boulardii treatment for acute diarrhea in children.
II. Design
Database search for randomised controlled and non-randomised trials (RCTs) investigating S. boulardii in childhood acute diarrhea.
Inclusion criteria for studies: RCTs with children aged 0 to 18 years with acute diarrhea ≤14 days.
III. Outcome
In this meta-analysis, the outcomes are:
- Duration of diarrhea
- Stool frequency on days 2 and 3
- Risk of diarrhea on days 3 and 4
IV. Results
A. Studies reviewed:
- n = 22 studies, 2440 participants (n = 1225 interventions and n = 1215 controls)
- Patients aged 1 month – 15 years
B. Outcomes:
compared to the control group (mean difference = -19.7 hours; 95% CI: -26.05 to -13.34; p <0.001).
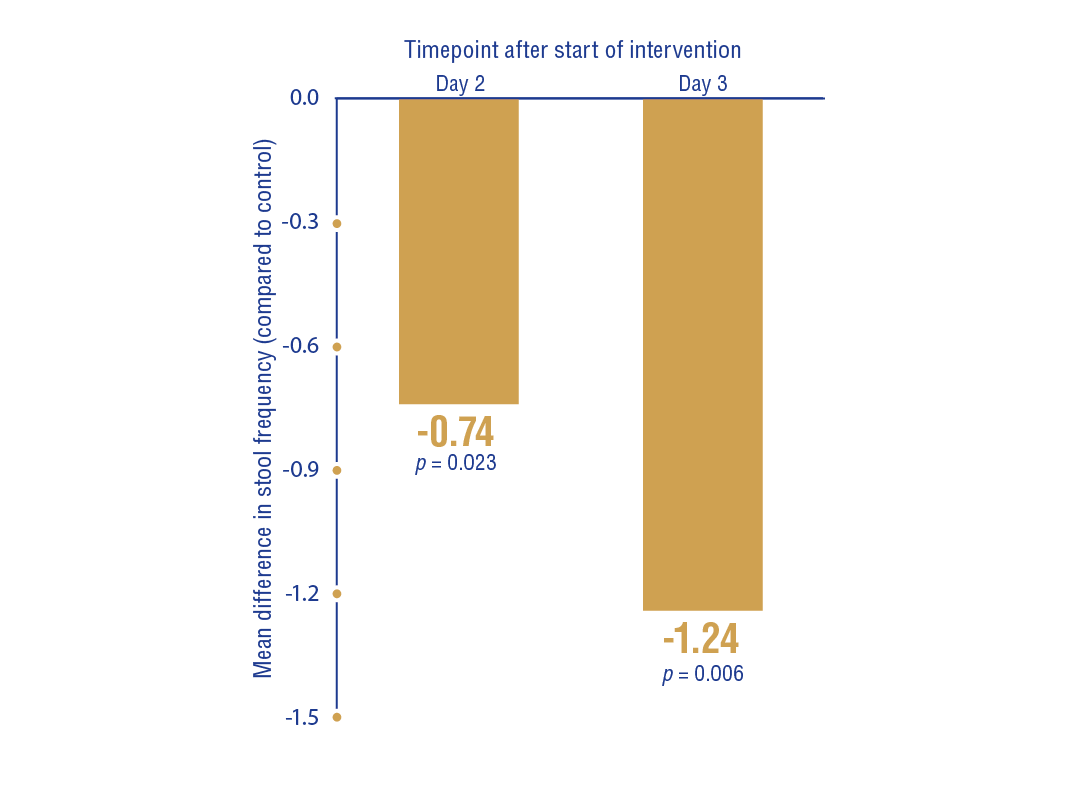
S. boulardii significantly reduced stool frequency on day 2 (MD: -0.74; 95% CI: -1.38 to -0.10; p = 0.023) and day 3 (MD: -1.24; 95% CI: -2.13 to -0.35; p = 0.006) compared to the control group (Figure 6).
(RR: 0.41; 95% CI: 0.27-0.60) and by 62% on day 4 (RR: 0.38; 95% CI: 0.24-0.59; p <0.01) compared to the control group.
V. Conclusion
Internal code : 20.65
References
- 01 . Szajewska H, Kołodziej M. Systematic review with meta-analysis: Saccharomyces boulardii in the prevention of antibiotic-associated diarrhoea. Alimentary Pharmacology and Therapeutics. 2015;42(7):793-801. doi:10.1111/apt.13344
- 02 . Zhou BG, Chen LX, Li B, Wan LY, Ai YW. Saccharomyces boulardii as an adjuvant therapy for Helicobacter pylori eradication: A systematic review and meta-analysis with trial sequential analysis. Helicobacter. 2019;24(5):1-16. doi:10.1111/hel.12651
- 03 . McFarland L V. Meta-analysis of probiotics for the prevention of antibiotic associated diarrhea and the treatment of Clostridium difficile disease. American Journal of Gastroenterology. 2006;101(4):812-822. doi:10.1111/j.1572-0241.2006.00465.x
- 04 . Szajewska H, Kołodziej M, Zalewski BM. Systematic review with meta-analysis: Saccharomyces boulardii for treating acute gastroenteritis in children-a 2020 update. Alimentary Pharmacology & Therapeutics. 2020;51(December 2019):678-688. doi:10.1111/apt.15659
- 05 . Feizizadeh S, Salehi-Abargouei A, Akbari V. Efficacy and safety of Saccharomyces boulardii for acute diarrhea. Pediatrics. 2014;134(1). doi:10.1542/peds.2013-3950
